Abstract
Introduction: Dasatinib is a potent tyrosine kinase inhibitor (TKI) of BCR-ABL . It is currently approved for the treatment of all phases of CML. Approved dose in the chronic phase is 100mg daily. This is however associated with notable side effects mainly myelosuppression and pleural effusion, leading to dose reductions/interruptions. Lower doses of dasatinib have been shown to be effective in elderly CML patients.
Objective: To evaluate the efficacy and toxicity profile of lower dose dasatinib, at 50 mg orally daily in patients with early CML-CP.
Methods: All patients presenting to our institution in early CML-CP were eligible to participate. Prior TKI therapy for up to 1 month was allowed. Responses were assessed according to the European LeukemiaNet guidelines (Baccarani et al. Blood 2013 122.872:884).
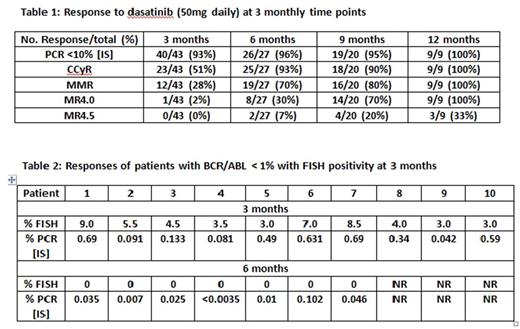
Results: From March 2016 to July 2017, 56 patients have been enrolled. Median age is 46 years (range 22-80). Patients categorized by Sokal risk are: low 34; intermediate 16 and high 6. Nine patients received prior TKI (for <= 30days): dasatinib 5; imatinib 3; nilotinib 1. Response to lower dose dasatinib is shown in Table 1.
At 3 months, 93% patients had achieved early molecular response (BCR-ABL PCR <10%) to therapy. Ten patients had BCR-ABL PCR < 1% (equivalent to CCyR) at 3 months but tested positive by FISH as shown in Table 2. At 6 months, 7 of these 10 patients that were FISH positive at 3 months, achieved CCyR (FISH 0%); of these 7 patients, 6 patients achieved a MMR. Three of the remaining 10 patients that were FISH positive at 3 months, have not yet reached (NR) the 6 month follow-up.
Nine patients had treatment interruption of up to 14 days in the 1st 3 months of therapy: gastrointestinal bleed 2; thrombocytopenia 3; transaminitis 2; renal dysfunction 2. None of these patients required dose modification and tolerated dasatinib once it was resumed.
Seven of these 9 patients with dose interruption have achieved early response to therapy while 2 patients have not yet reached the 3 month follow-up. Therapy was discontinued in 1 patient secondary to subdural hemorrhage (traumatic), while 1 patient at 6 months was deemed a failure due to lack of cytogenetic response and BCR/ABL PCR > 10%, requiring an increase in dasatinib dose to 100mg daily. None of the patients have developed pleural effusion so far.
Conclusion: Dasatinib at 50 mg daily dose is active and well-tolerated in newly diagnosed CML-CP. It should be further explored as a new potential dose-schedule option in CML.
Cortes: Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Teva: Research Funding; Pfizer: Consultancy, Research Funding; Sun Pharma: Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Pemmaraju: Cellectis: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Stemline: Consultancy, Honoraria, Research Funding; LFB: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria. Bose: Incyte Corporation: Honoraria. Thompson: Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kantarjian: Delta-Fly Pharma: Research Funding; Novartis: Research Funding; Amgen: Research Funding; ARIAD: Research Funding; Bristol-Meyers Squibb: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.